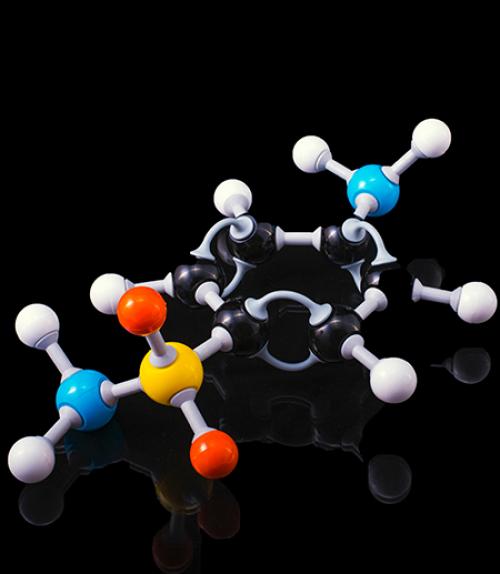
‘Roaming’ molecular fragments captured in real time
Sometimes atoms, like pets and adventuresome hikers, slip loose and wander off into the wild. Their final destination isn’t known, and their trajectory can be all over the map. It’s not so easy to track their path.
More news


Abruña honored for chemistry in the public interest
A&S Communications

Student spotlight: Bayu Ahmad
Cornell University Graduate School