New research from a multi-disciplinary team helps to illuminate the mechanisms behind circadian rhythms, offering new hope for dealing with jet lag, insomnia and other sleep disorders.
Using innovative cryo-electron microscopy techniques, the researchers have identified the structure of the circadian rhythm photosensor and its target in fruit flies (Drosophila melanogaster), one of the major organisms used to study circadian rhythms. The research, “Cryptochrome-Timeless Structure Reveals Circadian Clock Timing Mechanisms” published April 26 in Nature.
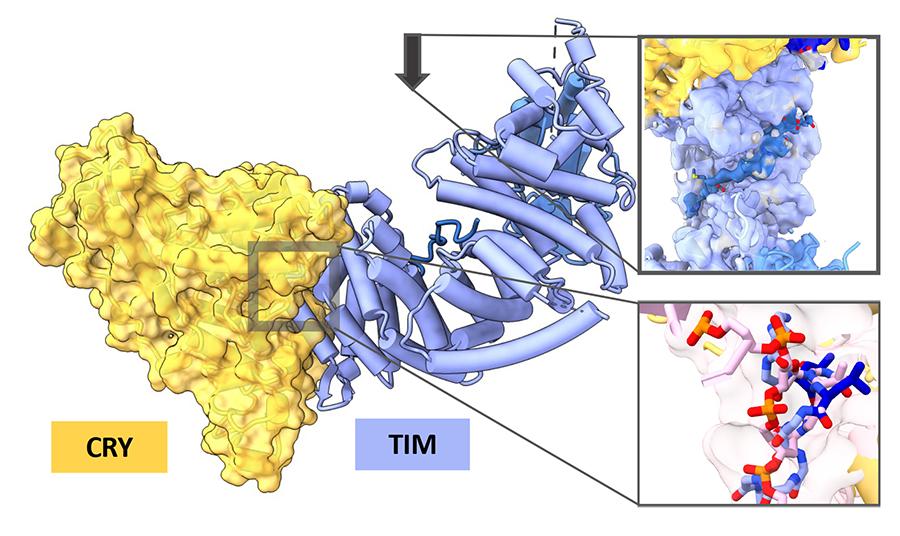
The research focused on fruit fly cryptochromes, key components of the circadian clocks of plants and animals, including humans. In flies and other insects, cryptochromes, activated by blue light, serve as the primary light sensors for setting circadian rhythms. The target of the cryptochrome photosensor, which bears the colorful name of “Timeless” (TIM), is a large, complex protein that could not previously be imaged and thus its interactions with the cryptochrome are not well understood.
Circadian rhythms work via “transcriptional translational feedback loops,” which are basically genetic feedback loops. According to the researchers, the TIM protein, along with its partner, the Period (PER) protein, act together to inhibit the genes that are responsible for their own production. With suitable delays between the events of gene expression and repression an oscillation in protein levels is established. Blue light changes the chemistry and structure of Cryptochrome’s flavin cofactor, which allows the protein to bind the TIM protein and inhibit TIM’s ability to repress gene expression and thereby reset the oscillation.
This oscillation represents the “the ticking of the clock and seems to be fairly unique to the circadian rhythm,” said senior author Brian Crane, the George W. and Grace L. Todd Professor and chair of chemistry and chemical biology in the College of Arts and Sciences.
Much of the hard work of the study went into figuring out how to produce the complex of cryptochrome-TIM so it could be studied, because TIM is such a large, unwieldy protein, said Crane. To achieve their results, first author Changfan Lin M.S. ’17, Ph.D. ’21 modified the cryptochrome protein to improve the stability of the cryptochrome-TIM complex and used innovative techniques to purify the samples, making them suitable for high-resolution imaging.
“These new methods allowed us to obtain detailed images of the protein structures and gain valuable insights into their function, said Lin, a Friedrich's Ataxia Research Alliance Postdoctoral Fellow at the California Institute of Technology. “By studying key proteins involved in fruit fly circadian rhythms, we uncovered the intricate molecular structures and mechanisms behind the interaction of light-sensitive protein Cry and regulatory protein Tim. This research not only deepens our understanding of circadian rhythm regulation but also opens up new possibilities for developing therapies targeting related processes.”
Shi Feng, a doctoral student in the field of biophysics, did much of the cry-electron microscopy work. “Cryo-EM requires a good deal of specialized knowledge from a variety of disciplines including physics, chemistry, and computer science,” said Feng. “Preparing the sample can be tricky, as every protein is different, and we have to optimize for specific samples, which can be time-consuming and requires experience. Processing and analyzing the data from Cryo-EM can take more than six months, and the disordered regions of Cry-Tim makes the processing even more challenging.”
Cristina C. DeOliveira, a doctoral student in the field of biochemistry and molecular and cell biology, was also a co-author of the paper. Feng and DeOliveira both currently work in the Crane Lab.
“It’s a testament to the benefits of the Cornell graduate field system, that all three co-authors, working together in my lab, synergized their research efforts despite coming from different backgrounds and interests,” said Crane.
One unexpected result from the study sheds light on how DNA damage is repaired in a cell. Cryptochromes are closely related to a family of enzymes involved in repairing damage to DNA, called photolyases, which use light to repair cross-linked base pairs in DNA that form from ultraviolet light exposure. Although it’s long been recognized that cryptochrome and photolyase is nearly identical, they have distinct biological functions and it wasn’t clear what connection they could have.
“What we find from this study is that the way that that the cryptochrome recognizes the TIM protein structurally mimics the way that the photolyase recognizes DNA substrates in order to repair the damage,” said Crane. “It explains why these families of proteins are closely related to each other, even though they're doing quite different things -- they're making use of the same molecular recognition in different contexts.”
The study also offers an explanation for the genetic variation of flies that allow them to adapt to higher lattitudes, environments where days are shorter in the winter and it's cooler. These flies have more of a certain genetic variant that involves a change in the TIM protein, and it wasn’t clear why the variation could help them. The researchers found that because of how the cryptochrome binds TIM the variation reduces the affinity of TIM for the cryptochrome. The interaction between the proteins is then modulated and the ability of light to reset the oscillation is changed, thus altering the circadian clock and extending the period of the fly’s dormancy, which helps it survive the winter.
“Some of the interactions that we see here in the fruit fly can be mapped onto human proteins,” said Crane. “This study may help us understand key interactions between components that regulate sleep behavior in people, such as how the critical delays in the basic timing mechanism get built into the system.”
Another exciting finding, said Lin, was the discovery of an important structural area in TIM, called the "groove," which helps explain how TIM enters the cell nucleus. Previous studies had identified some factors involved in this process, but the exact mechanism remained unclear. “Our research provided a clearer understanding of this phenomenon,” said Lin.
The research used equipment at the cryo-electron microscopy center, established thanks to the College of Arts and Sciences and Provost Michael Kotlikoff, said Crane. “A lot of groups on campus are really starting to benefit from it. We’re discovering new things with it that we couldn't have done before. We've also had great collaborations with the Center for Materials Research and faculty in the College of Engineering, expert microscopists who’ve been a great help with the instrument.”