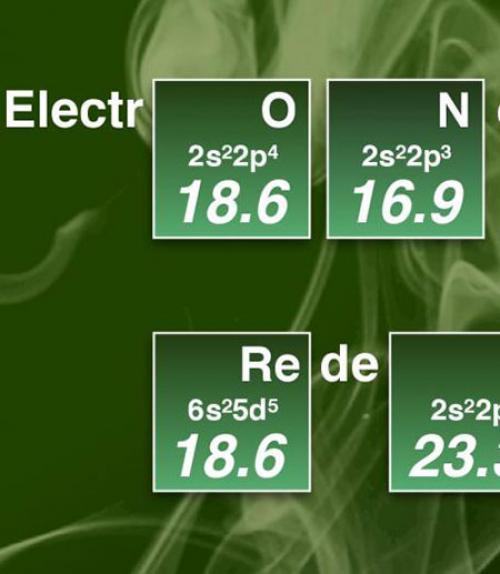
Chemists develop new scale for electronegativity
Electronegativity is one of the most well-known models for explaining why chemical reactions occur. Used daily by chemists and materials researchers all over the world, the theory of electronegativity is used to describe how strongly different atoms attract electrons. In a new paper, researchers have redefined the concept with a more comprehensive electronegativity scale.
More news


Abruña honored for chemistry in the public interest
A&S Communications

Student spotlight: Bayu Ahmad
Cornell University Graduate School